Abstract
Introduction: Idelalisib (Zydelig®, IDELA) is a PI3Kδ inhibitor approved for treatment of relapsed or refractory (R/R) follicular lymphoma and in combination with anti-CD20 for R/R chronic lymphocytic leukemia (CLL). While IDELA is efficacious, emergence of adverse events (AEs) such as diarrhea, colitis, and transaminitis may limit its long-term administration. We hypothesize that IDELA dose interruption followed by re-initiation as a strategy to manage AEs will permit patients to remain on drug longer and improve clinical outcomes.
Objective: To evaluate the impact of IDELA treatment interruption on duration of IDELA therapy (DoT), progression-free survival (PFS), and overall survival (OS) in patients with R/R indolent non-Hodgkin's lymphoma (iNHL) and R/R CLL.
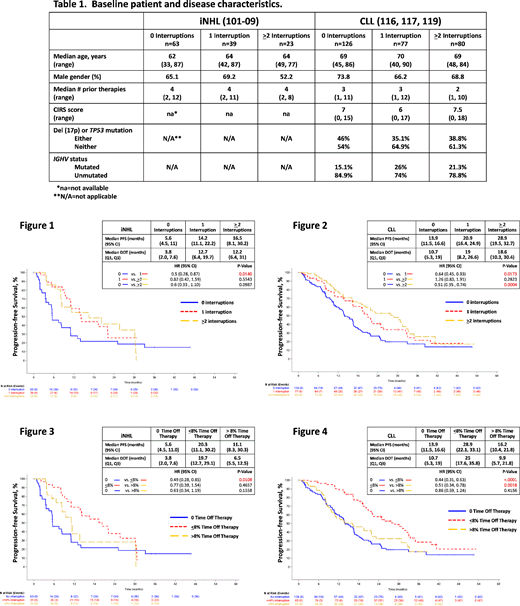
Methods: Using clinical outcomes data from Gilead-sponsored trials of patients with R/R iNHL treated with IDELA (101-09, N=125, Gopal et al., N. Engl. J. Med. 2014; 370:1008) or with R/R CLL treated with IDELA + anti-CD20 (312-0116/0117, N=110, Furman et al., N. Engl. J. Med. 2014; 370:997 and 312-0119, N=173, Jones et al., Lancet Haematol. 2017; 4:e114), the effect of IDELA interruption on DoT, PFS, and OS was evaluated retrospectively. Interruption was defined as missing at least one treatment day due to an AE. Patients were stratified according to number of treatment interruptions (0, 1, or ≥2) and by percentage of time off therapy (0, ≤8%, and >8%). The 8% threshold was based on the median (m) percentage of time off therapy in patients with ≥1 drug interruption. PFS and OS were estimated using the Kaplan-Meier method and were compared using a stratified log-rank test.
Results: Among patients with R/R iNHL, 49.6% interrupted therapy, with 31.2% experiencing 1 interruption and 18.4% ≥2 interruptions. Among patients with R/R CLL, 55.5% interrupted therapy, with 27.2% experiencing 1 interruption and 28.3% ≥2 interruptions. Baseline patient and disease characteristics were similar among the various treatment interruption groups (Table 1). The most common AEs (any grade) leading to any treatment interruption were diarrhea (iNHL, 14.5%; CLL, 28%), cytopenias or febrile neutropenia (iNHL, 4.8%; CLL, 22.3%), hepatic toxicity (elevated transaminases, alkaline phosphatase, or bilirubin; iNHL, 4.8%; CLL, 16.6%), and pneumonia (iNHL, 9.7%; CLL, 9.6%). iNHL and CLL patients without treatment interruption tended to have a shorter total DoT (iNHL, 3.8 months; CLL, 10.7 months) than patients with either 1 (iNHL, 12.7 months; CLL, 19 months) or ≥2 (iNHL, 12.2 months; CLL, 18.6 months) interruptions. iNHL and CLL patients with any treatment interruption achieved a longer PFS and OS than patients with no interruption (iNHL, mPFS=5.6 months, 14.2 months, and 16.5 months for 0, 1, and ≥2 interruptions, respectively [Fig. 1] and mOS=not reached (NR) for all groups; CLL, mPFS=13.9 months, 20.9 months, and 28.9 months [Fig. 2] and mOS=33.6 months, NR, and 47.4 months for 0, 1, and ≥2 interruptions, respectively). To account for differences in DoT across the treatment interruption groups, we compared PFS and OS based on percentage of time off therapy (no time off therapy, ≤8% time off therapy, and >8% time off therapy). Patients who interrupted with time off therapy ≤8%, but not >8%, had improved PFS and OS compared to patients who did not interrupt (iNHL, mPFS=5.6 months, 20.3 months, and 11.1 months for no, ≤8%, and >8% time off therapy, respectively [Fig. 3] and mOS=NR for all groups; CLL, mPFS=13.9 months, 28.9 months, and 16.2 months [Fig. 4] and mOS=33.6 months, 47.4 months, and NR for no, ≤8%, and >8% time off therapy, respectively).
Conclusion/Discussion: In these clinical trials, approximately half the patients interrupted IDELA therapy due to AEs. Patients who interrupted therapy had a longer DoT and achieved longer PFS and OS than patients who did not interrupt. While limited time off therapy appeared to confer a clinical benefit, additional time off therapy (defined by an 8% threshold) did not provide a benefit beyond that achieved with no interruption. These findings suggest that management of IDELA AEs via treatment interruption and clinically appropriate re-initiation may benefit some patients by improving tolerability and prolonging PFS and OS. Additional analyses evaluating the influence of dose reductions are ongoing. The impact of IDELA treatment interruption on clinical outcomes should be confirmed with prospective clinical studies.
Ma:Acerta: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees. Ye:Gilead Sciences, Inc.: Employment, Equity Ownership. Xing:Gilead Sciences, Inc.: Employment. Brubaker:Gilead Sciences: Employment, Equity Ownership. Roudet:Gilead Sciences, Inc.: Employment. Ruzicka:Gilead Sciences, Inc.: Employment. Wagner-Johnston:ASTEX: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; JUNO: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Celgene: Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal